Assessing skin corrosion is crucial for the safety of chemical, cosmetic, detergent, and pharmaceutical products. OECD Guideline 431 defines an in vitro method for determining whether a substance or mixture is corrosive to the skin, without resorting to animal testing. Internationally recognized, this method is an integral part of the regulatory testing strategies required by European and global authorities, particularly within the framework of the REACH Regulation and the CLP Regulation on the classification and labeling of substances.
Table of Contents
OECD 431: in vitro method
Definition of skin corrosion
Skin corrosion refers to the development of irreversible damage to the skin, leading to tissue destruction and visible necrosis. According to the Globally Harmonized System of Classification and Labelling (GHS), a substance is considered corrosive when it causes such damage after a single application to the skin. Unlike simple irritation, skin corrosion has permanent effects that require rigorous identification before any product is placed on the market.
OECD 431 Test Objective
The OECD 431 test 's main objective is to distinguish corrosive substances from non-corrosive substances. It also allows, in certain cases, for the sub-categorization of corrosive products based on their intensity.
- Category 1A: highly corrosive substances with rapid effects.
- Categories 1B and 1C: corrosive substances with less rapid but still irreversible effects.
Thanks to the use of in vitro models, this test provides a reliable alternative to historical animal testing, notably the OECD 404 test conducted on rabbits. It fully aligns with the 3Rs principles (replace, reduce, refine animal testing).
Regulatory and ethical importance
The adoption of the OECD 431 test is driven by two key issues. Firstly, it meets the regulatory requirements set by international legislation such as REACH in Europe, which mandates that manufacturers characterize the hazards of their chemical substances. Secondly, it aligns with an ethical approach aimed at reducing the use of laboratory animals. Today, results obtained through OECD 431 are recognized by regulatory authorities in all OECD member countries thanks to the principle of mutual acceptance of data.
In summary, OECD 431 constitutes a scientific reference method for assessing the corrosive potential of a product, while contributing to the protection of human health and the reduction of animal testing.
OECD 431 Scientific Principle of the Test
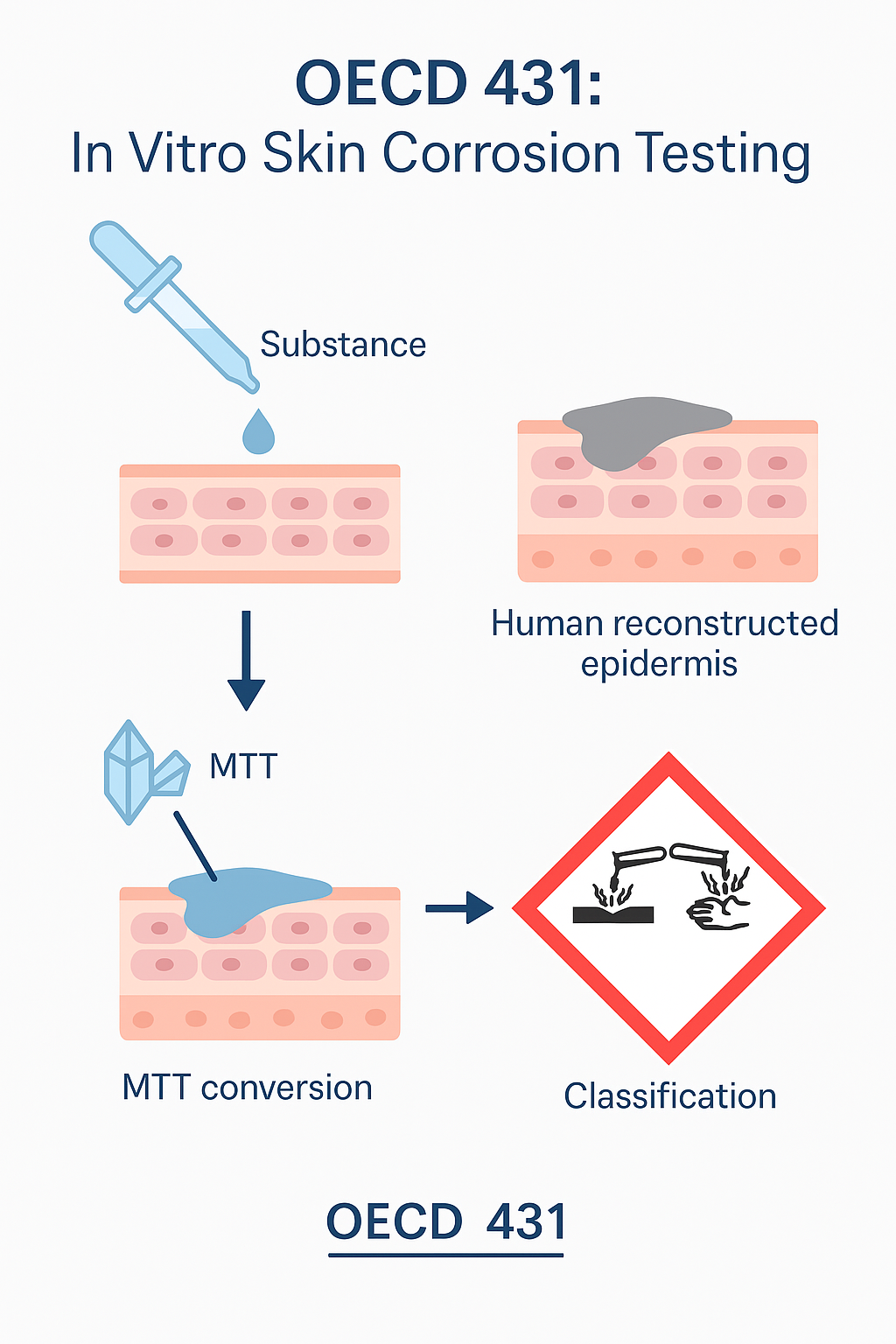
The OECD 431 test relies on the use of in vitro models of reconstituted human epidermis, allowing for the reproduction of the main physiological and biochemical characteristics of human skin. This method is designed to assess the ability of a chemical substance to cause skin corrosion, that is, irreversible destruction of skin tissue.
The reconstituted human epidermis model
The assay uses a three-dimensional human epidermis, created from cultured human keratinocytes to replicate the different skin layers. This model includes the stratum corneum, which acts as a protective barrier, as well as the more sensitive underlying layers. Thanks to this structure, the reconstructed epidermis closely mimics real skin and allows for the measurement of the diffusion and cytotoxic effect of the tested substances.
The role of the MTT test in measuring cell viability
The OECD 431 test is based on the conversion of the vital dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) by viable cells. Living cells transform this dye into an insoluble compound called formazan, which is blue. The amount of formazan produced is directly proportional to the number of viable cells. A significant decrease in cell viability therefore indicates a corrosive effect of the tested substance.
To ensure the reliability of the results, each trial is conducted with multiple replicates and includes positive and negative controls. This verifies that the reconstituted epidermis reacts as expected and that the substance has been properly evaluated under controlled conditions.
The viability thresholds defined by the OECD
The OECD has established precise thresholds for cell viability to classify a substance:
- If cell viability is greater than 50% after 3 minutes of exposure and greater than 15% after 60 minutes, the substance is considered non-corrosive.
- If viability falls below these thresholds, the substance is classified as corrosive.
- Depending on the level of cytotoxicity observed, it is possible to sub-categorize the substance into GHS classes 1A, 1B or 1C.
These criteria ensure a harmonized classification at the international level, which is essential for placing chemical and cosmetic products on the market.
Management of interfering substances
Certain chemicals can bias the test results. For example, naturally colored products can mask the reading of the MTT dye, while strongly reducing or oxidizing substances can interfere with the chemical reaction. In these specific cases, alternative procedures, such as high-performance liquid chromatography (HPLC), are planned to confirm the results.
The scientific advantages of the method
The OECD 431 test has several major advantages:
- It is based on a biological model close to human reality, increasing the relevance of the results compared to older animal tests.
- It allows for the standardization of experimental conditions through the use of validated models and precise thresholds.
- It promotes a significant reduction in the use of animal testing, in accordance with the ethical principles of the 3Rs.
By combining scientific reliability, regulatory compliance and respect for ethical considerations, the OECD 431 test principle has now become an essential method for assessing skin corrosion.
Methods and models validated by the OECD
The OECD 431 test is not limited to a single experimental protocol. It encompasses several validated methods, all of which use reconstituted human epidermis models, but developed by different laboratories or manufacturers. These methods have been evaluated and approved by the OECD to ensure their reliability and reproducibility at the international level.
Validated human epidermis models
Among the models marketed and recognized by the OECD are:
- EpiDerm™ (MatTek, USA) : a widely used model based on human keratinocytes grown in three dimensions.
- EpiSkin™ (L'Oréal, France) : one of the first validated models, faithfully reproducing the histological characteristics of the human epidermis.
- SkinEthic™ RHE (L'Oréal, France) : reconstituted skin model used for various toxicological tests, including corrosion and skin irritation.
- epiCS® (CellSystems, Germany) : reconstituted epidermis certified in accordance with OECD guidelines.
- LabCyte EPI-MODEL24 (J-TEC, Japan) : model recognized for its robustness and its use in international regulatory tests.
These models are available as ready-to-use kits and allow laboratories to carry out standardized tests, comparable from one country to another.
Scope of applicability of OECD methods 431
The OECD 431 test is applicable to a wide range of substances:
- Solids, powders and crystals.
- Liquids, including aqueous solutions and solvents.
- Semi-solids such as pastes or gels.
- Waxes and complex mixtures.
However, some limitations must be mentioned. OECD 431 methods are not yet validated for evaluating gases, aerosols, and certain highly volatile formulations. In these cases, other tests or complementary approaches should be considered.
The subcategorization of corrosive substances
One of the strengths of the OECD 431 test is its ability to distinguish not only corrosive from non-corrosive substances, but also to specify their degree of hazard. Thanks to cell viability thresholds, it is possible to direct the classification towards:
- Category 1A : severe corrosivity, rapid effects on skin.
- Categories 1B and 1C : less rapid corrosivity, but still causing irreversible damage.
This precision is essential for the classification and labeling of chemical products according to the CLP regulation and the GHS.
Comparison with other OECD tests
The OECD 431 test is part of a broader set of in vitro guidelines for skin safety assessment:
- OECD 439 : a skin irritation method also using reconstituted human epidermis. It allows for the differentiation of irritant substances (category 2) from unclassified substances.
- OECD 435 : test on artificial sealing membrane, based on the measurement of the penetration time of the substance.
- OECD 430 : Alternative method of skin corrosion using a perfusion system.
By combining these different approaches, laboratories have a complete strategy for assessing both corrosion and skin irritation, without resorting to animal testing.
Standardization and international acceptance
All methods validated under OECD 431 follow rigorous protocols that guarantee high reproducibility. Mutual acceptance of data between OECD member countries is a major advantage: a study conducted according to this guideline in a certified laboratory is recognized as valid in all member countries, thus avoiding duplicate testing and reducing costs for industry.
In summary, the methods and models validated by OECD 431 represent a major advance in the toxicological assessment of skin corrosion. They offer a robust and standardized alternative, adapted to the needs of industry and international regulatory requirements.
Are you looking for an analysis?

The regulatory framework surrounding the OECD 431 test
The OECD 431 test plays a central role in international regulations concerning the classification and labeling of chemical substances. By allowing the assessment of skin corrosion without resorting to animal testing, it meets both the legal obligations of industry and the ethical expectations of society.
The main European regulations
Several European laws mandate or recognize the use of OECD 431:
- REACH Regulation (EC No. 1907/2006) : This regulation requires manufacturers and importers of chemical substances to assess and communicate their potential hazards, including skin corrosion. The OECD 431 test is recognized as the reference method for meeting these requirements.
- CLP Regulation (EC No. 1272/2008) : relating to the classification, labelling and packaging of substances and mixtures, it requires the determination of the skin hazard of products before they are placed on the market.
- Biocidal Products Directive (EU No. 528/2012) : active substances and biocidal products must be subjected to skin safety tests, for which OECD 431 is accepted.
- Detergents Regulation (EC No. 648/2004) : it provides for tests on cleaning and household products to guarantee their safety of use.
- Cosmetics Regulation (EC No. 1223/2009) : requires verification of the skin safety of cosmetic ingredients and prohibits animal testing, reinforcing the importance of in vitro methods such as OECD 431.
OECD Mutual Acceptance of Data
One of the major advantages of the OECD 431 test is that it benefits from the principle of Mutual Acceptance of Data (MAD) . This means that a test carried out according to this guideline in one OECD member country is recognized by all other member countries. For manufacturers, this translates into:
- a reduction in costs associated with test duplication,
- a simplification of the international marketing of products,
- a harmonization of classification and labeling criteria.
The role of GLP, ISO 17025 and COFRAC certified laboratories
For OECD 431 test results to be legally valid, they must be produced in laboratories that meet quality and competence standards.
- Good Laboratory Practices (GLP) guarantee the traceability, reliability and integrity of the data generated.
- ISO 17025 standard defines the general competence requirements for testing and calibration laboratories.
- COFRAC accreditation ensures that tests are carried out according to the strictest European standards and guarantees their recognition by the authorities.
These certifications enhance the scientific credibility of the tests and reassure manufacturers about the regulatory validity of their results.
Use of results in safety data sheets
The results of the OECD 431 test are directly used in the drafting of Safety Data Sheets (SDS) . These regulatory documents, mandatory for the marketing of chemical substances and mixtures, must clearly indicate whether the product is corrosive or not. The OECD 431 classification also determines the hazard statements, pictograms, and precautionary statements that appear on labels and packaging.
Industrial applications of the OECD 431 test
The OECD 431 test is not just a scientific research method; it is a regulatory and operational tool applicable to many industrial sectors. It helps ensure product safety, protect consumers, and meet legal requirements before any product is placed on the market.
Applications in the cosmetics industry
In the cosmetics sector, ingredient safety is an essential regulatory requirement. Since the ban on animal testing in Europe, in vitro methods such as OECD 431 have become the standard.
- Manufacturers use this test to verify that active ingredients or excipients do not present a risk of skin corrosion.
- The results guide the formulation of products such as creams, lotions, shampoos or gels, in order to ensure safe application for consumers.
- Positive or negative results also determine the mandatory information on the labels, in accordance with EC Regulation 1223/2009.

Applications in the detergent and household products industry
Household and industrial products, such as cleaners and degreasers, often contain powerful chemical agents. The OECD 431 test can be used to verify:
- If these products are corrosive to human skin,
- In which danger category should they be classified?
- what precautions for use should be mentioned on their packaging (pictograms, hazard statements, precautionary advice).
This test is therefore essential to meet the requirements of the detergents regulation (EC No. 648/2004) and to ensure the safety of users.
Applications in the chemical and pharmaceutical industries
Pure chemicals and pharmaceuticals must be evaluated before being authorized for marketing. The OECD 431 test is used:
- to determine the corrosivity of the base substances used in manufacturing,
- to properly classify and label pharmaceutical products or synthetic intermediates,
- to anticipate the risks associated with skin exposure of operators in the laboratory or in production.
In the case of topical pharmaceutical products (creams, gels, ointments), this test ensures that the formulations meet the safety criteria for the skin.
Applications in agrochemicals and biocides
Plant protection products and biocides must undergo rigorous toxicological evaluations before any registration. The OECD 431 test is used for:
- check that the active substances or adjuvants do not cause skin corrosion,
- determine safe usage conditions for farmers, gardeners or technicians,
- to provide the data necessary for the regulatory files required by the Biocidal Products Directive and the regulations on plant protection products.
Cross-cutting benefits for manufacturers
Beyond sector-specific differences, OECD 431 offers several common advantages to manufacturers:
- Regulatory compliance : a test recognized by all OECD member authorities.
- Cost reduction : a single study allows for international recognition.
- User protection : products placed on the market are correctly classified and labeled.
- Ethical valuation : the use of an in vitro method reinforces the image of social and environmental responsibility of companies.
Ultimately, OECD 431 is a crucial link in the safety chain for chemical, cosmetic, pharmaceutical, and agrochemical products. It contributes directly to the protection of consumers and workers while facilitating regulatory compliance for businesses.
Integrated approaches: IATA and testing strategy
The OECD 431 test does not operate in isolation. It is part of a broader skin safety assessment strategy called the Integrated Testing and Evaluation (IATA) approach. This approach combines several in vitro methods, existing data, and sometimes modeling to provide a comprehensive and reliable assessment of the risk of skin corrosion or irritation.
Top-down and bottom-up logic
Two complementary strategies are defined to guide the use of the OECD 431 test:
- Top-down approach : when a substance is suspected of being corrosive, a skin corrosion test (OECD 431 or OECD 435) is performed first. If the results confirm corrosivity, no further testing is necessary.
- Bottom-up approach : When a substance is assumed to be non-corrosive, the first test performed is the skin irritation test (OECD 439). If the substance is irritating, the process stops. If it is not, a skin corrosion test is then performed to rule out any risk of irreversible damage.
This gradual strategy allows for optimization of the number of tests performed and for obtaining a precise classification of the substance tested.
Complementarity with other in vitro methods
OECD 431 is part of a set of guidelines developed to replace animal testing:
- OECD 439 : specifically assesses skin irritation and distinguishes irritant substances (category 2) from unclassified substances.
- OECD 435 : relies on an artificial sealing membrane that mimics the skin barrier and measures the penetration time of a substance.
- OECD 430 : uses a perfusion system to study skin corrosion.
By using these methods in combination, laboratories can obtain a complete view of skin hazard and avoid duplication.
Reduction of animal testing and the principles of the 3Rs
IATA and OECD 431 are fully aligned with the 3Rs :
- Replace animal testing with reliable alternatives.
- Reduce the number of animals used in experiments.
- Refining methods to limit animal suffering.
The use of reconstituted human epidermis models represents a major ethical advance, while providing results more relevant to humans than historical animal models.
OECD 431 analyses with YesWeLab: your partner for regulatory testing
Performing an OECD 431 test requires technical expertise, a thorough understanding of international guidelines, and strict adherence to quality standards. In this area, YesWeLab positions itself as a strategic partner for manufacturers seeking reliability, speed, and regulatory compliance.
Multi-sector expertise at the service of manufacturers
YesWeLab collaborates with a network of over 200 partner laboratories in France and Europe, specializing in various fields. Thanks to this diversity, the platform is able to meet the needs of a wide range of sectors:
- Cosmetics industry : verification of the safety of ingredients and formulations in accordance with EC Regulation 1223/2009.
- Chemical and pharmaceutical industry : analysis of pure substances or complex formulations to ensure their compliance with the REACH regulation.
- Detergent and household products industry : classification of corrosive products to protect users,
- agrochemical and biocides sector : toxicological evaluation of active substances before approval.
This multidisciplinary approach allows YesWeLab to support companies throughout their go-to-market process.
Guaranteed compliance with ISO 17025, COFRAC and GLP/GLP standards
All tests offered via YesWeLab, including the OECD 431 test, are carried out in laboratories that comply with Good Laboratory Practices (GLP) and are accredited according to international standards:
- ISO 17025 : guaranteeing technical competence and reliable results,
- COFRAC : ensuring official recognition in France and at the European level,
- GLP/GLP : guaranteeing the traceability, integrity and scientific quality of the data obtained, essential for submitting them to regulatory authorities.
These accreditations allow manufacturers to easily integrate test results into their regulatory files and safety data sheets.
A digital platform to simplify the management of analyses
YesWeLab has developed a unique solution that centralizes the management of laboratory analyses:
- Analysis search : an online catalogue with more than 10,000 services, including toxicological tests such as OECD 431.
- Sending samples : a simplified logistics process that reduces delays and avoids handling errors.
- Access to results : a secure digital platform that allows you to view and download test reports in complete transparency.
Thanks to this system, manufacturers gain in efficiency and benefit from complete traceability of their samples and results.
Concrete benefits for customers
Using YesWeLab for an OECD 431 test offers several advantages:
- a saving of time thanks to the simplification of administrative and technical procedures,
- a reduction in costs by avoiding duplication of tests through the mutual acceptance of OECD data,
- guaranteed compliance with European and international regulatory requirements,
- personalized support from dedicated experts who guide companies in choosing the relevant analyses.
A trusted partner for your regulatory testing
YesWeLab goes beyond simply connecting clients with laboratories; it acts as a true partner. The company is committed to providing solutions tailored to each client's needs, leveraging a strong network and proven expertise in key sectors: cosmetics, food processing, chemicals, pharmaceuticals, and the environment.
By choosing YesWeLab to carry out your OECD 431 tests, you benefit from an integrated solution combining scientific rigor, regulatory compliance and digital innovation.